Physical Chemistry
Phase rule
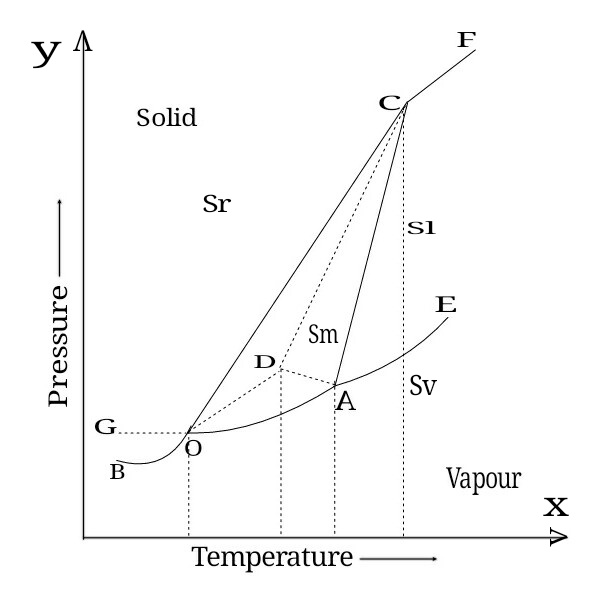
One component system
2] Sulphur System
Sulphur exists in four phases ie rhombic sulphur, monoclinic sulphur, liquid sulphur and vapour sulphur.
Sulphur rhombic and sulphur monoclinic are allotropes of sulphur.
Sr - Rhombic sulphur
Sl - Liquid sulphur
Sm - Monoclinic sulphur
Sv - sulphur vapours
In phase diagram of sulphur system we have
Six Stable curves (OB,OA,AE,OC,AC,CF)
Four areas (BOCF,OAC,FCAE,BOAE)
Four metastable curve (GO,OD,DA,DC)
Three triple point (O,A,C)
Point O ( T=95.6°C and P=0.006mmHg)
On point 'O' Three phases are in equilibrium ie
Sr ⇌ Sm ⇌ Sv
Point A (T=120°C and P=0.04mmHg)
On point A Three phases are in equilibrium ie
Sm ⇌ Sl ⇌ Sv
Point C (T=151°C and 1288 atm)
On point C Three phases are in equilibrium ie
Sr ⇌ Sm ⇌ Sl
Metastable triple point (D)
(T=115°C and P=0.03mmHg)
On the point D three phases are in equilibrium ie
Sr ⇌ Sl ⇌ Sv
By applying phase rule for triple point and metastable triple point we get
F=C-P+2 (C=1 and P=3)
F=1-3+2
F=0
Hense the system on triple points and metastable triple point is inonvariant or invariant or zerovariant.




Comments
Post a Comment